Health Reporter
The Medicines Control Authority of Zimbabwe (MCAZ) has issued a Class II recall for a specific batch of YAZ Plus contraceptive tablets due to a quality issue that could compromise the medication’s effectiveness.
The affected batch, identified as WEW961J, was manufactured by Bayer (Pty) Ltd in South Africa.
Details of the Quality Issue
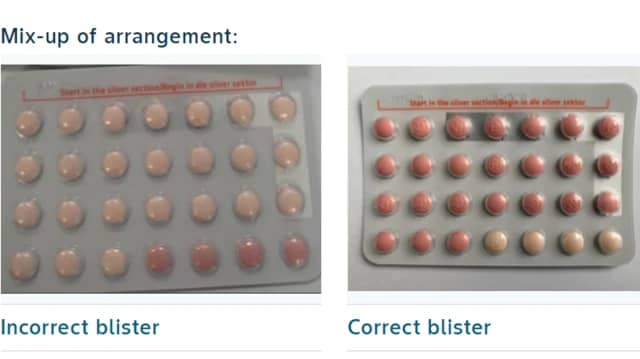
The recall was prompted by an incorrect configuration of active and inactive hormone tablets in the affected packs. Specifically, the faulty batch contains 24 light orange hormone-free tablets and four pink hormone tablets, instead of the correct formulation of 24 pink hormone tablets and four light orange hormone-free tablets. This discrepancy poses a risk of unintended pregnancies due to potential loss of efficacy .
Consumer Guidance
MCAZ has advised consumers to:
- Check the batch number on their YAZ Plus packets.
- Stop using the medication immediately if it belongs to the affected batch.
- Return the defective packs to pharmacies for a replacement or refund.
- Consult healthcare professionals for further guidance.
Instructions for Healthcare Providers
Healthcare professionals, hospitals, pharmacies, and wholesalers in possession of the defective batch are instructed to quarantine and return the affected units to distributors or Bayer (Pty) Ltd. This action is part of the recall process to ensure public safety.
Conclusion
The MCAZ emphasizes that this recall is a precautionary measure aimed at protecting public health from the potential ineffectiveness of the affected contraceptive tablets. Consumers are urged to take this matter seriously and follow the recommended steps to ensure their health and safety.
Zim GBC News©2024